TRANSFORMS
Study design
Double-blind, randomized, double-dummy, active-controlled, one-year Phase 3 study in adults with RRMS
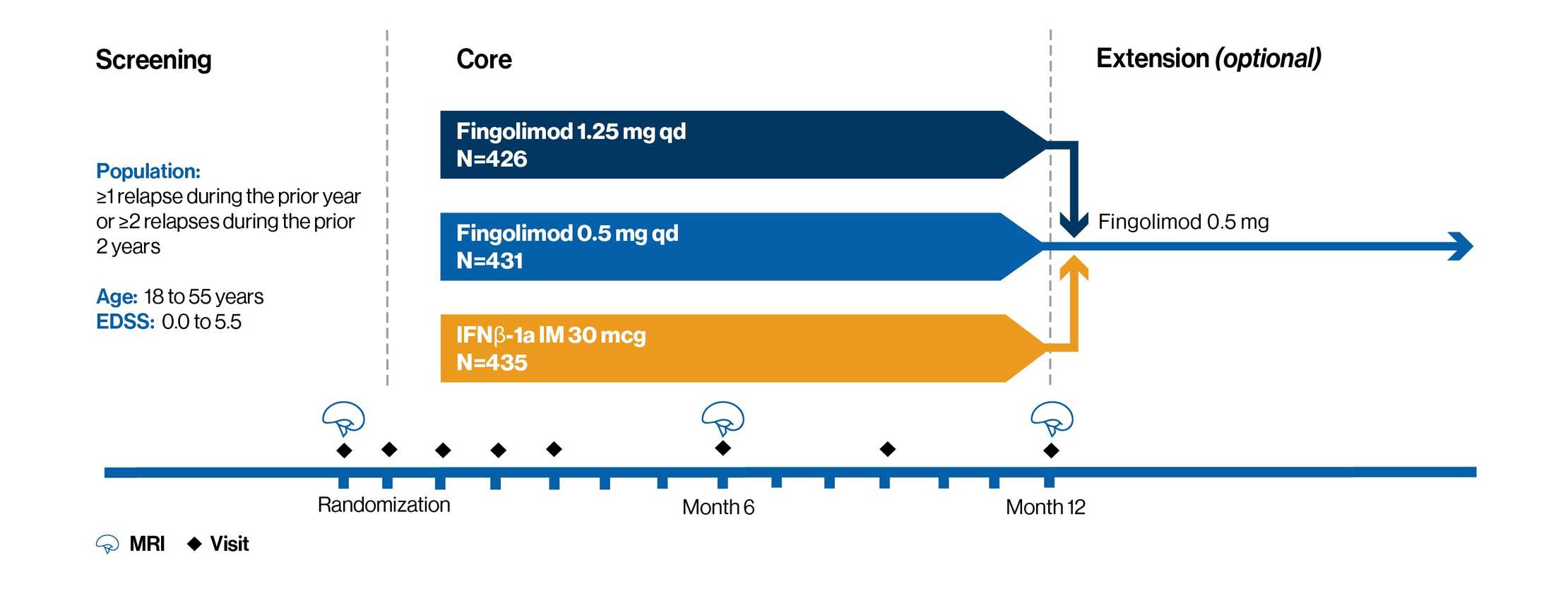
A 1-year, randomized, double-blind, double-dummy, active-controlled (IFNβ-1a IM), phase 3 study of 1,292 adults with RRMS
Patients were randomly assigned to receive a once-daily dose of fingolimod 0.5 mg (n=431) or 1.25 mg (n=426) or IFNβ-1a IM injection at a weekly dose of 30 μg (n=435) for 1 year
Patient baseline characteristics:
1. Between 18 and 55 years of age
2. A diagnosis of RRMS with at least 1 documented relapse during the previous year or at least 2 documented relapses during the previous 2 years
3. A score of 0.0 to 5.5 on the EDSS. Median score at baseline was 2.0
4. Previous therapy with either any type of interferon-beta or glatiramer acetate was not a criterion for exclusion
Primary end point: the primary objective was to demonstrate that fingolimod 0.5 mg was superior to IFNβ-1a in terms of ARR in patients with RRMS treated for up to 12 months
Two key secondary end points: number of new or newly enlarged hyperintense lesions on T2-weighted MRI scans at 1 year and the time to 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5) sustained for 3 month
AE profile of fingolimod versus interferon over 1 year: TRANSFORMS Core study1

AE profile of fingolimod over 4.5 years: TRANSFORMS Extension study2**

**All patients received fingolimod 0.5 mg in the extension
