LONGTERMS
The LONGTERMS study evaluated the safety and efficacy of fingolimod in patients with relapsing MS with up to 14 years of exposure. This Phase 3b, open-label extension study included patients aged ⩾18 years with a confirmed RMS diagnosis who completed previous Phase 2/3/3b core/extension studies of fingolimod
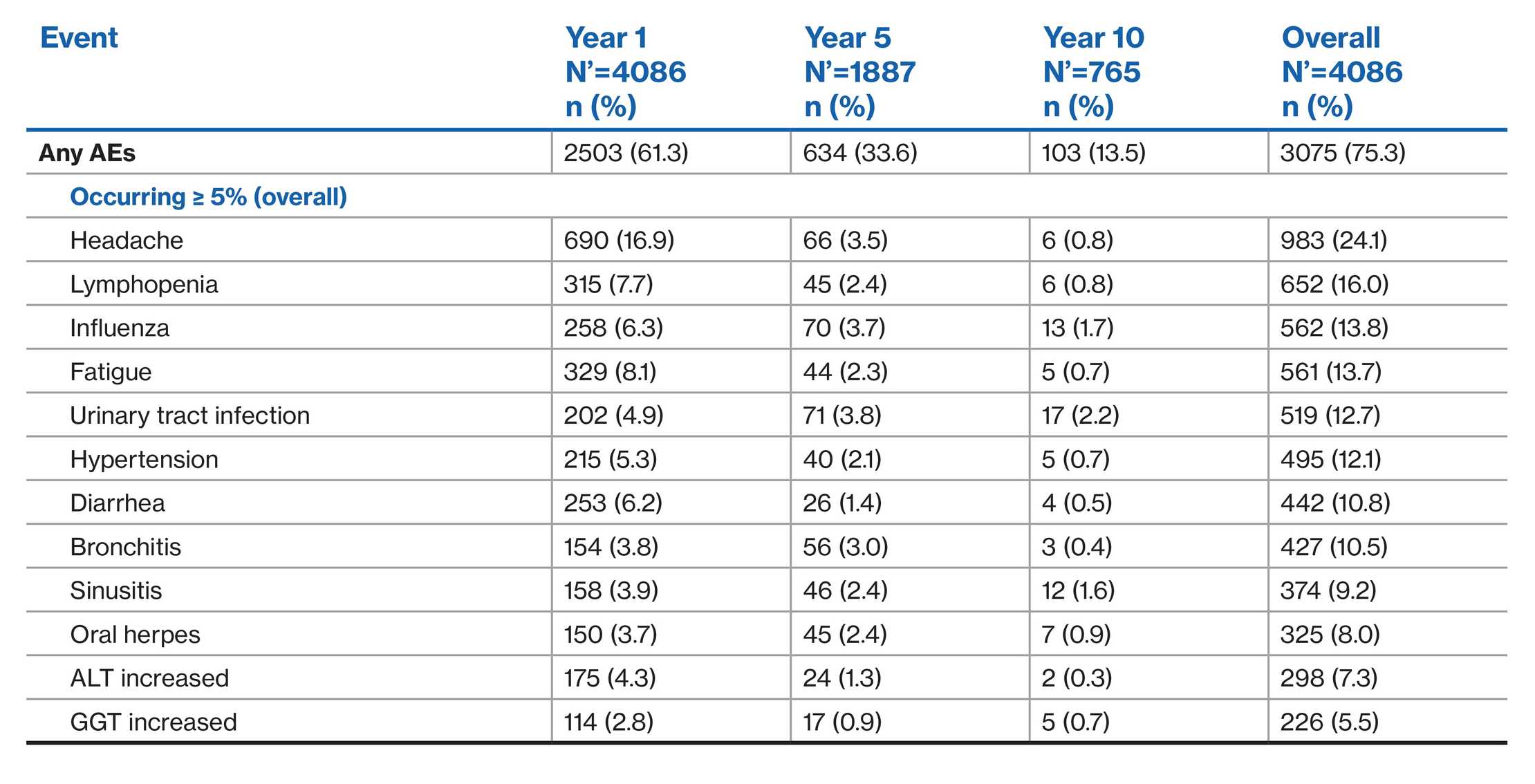
AEs profile of fingolimod over 10 years: LONGTERMS study1