REALMS
REALMS is a retrospective study based on the analysis of medical records in order to collect real-world evidence for the efficacy and safety of fingolimod in RRMS patients (N=275) from Portuguese MS centers during the 3-year follow-up
Adverse events by year after initiating fingolimod treatment1
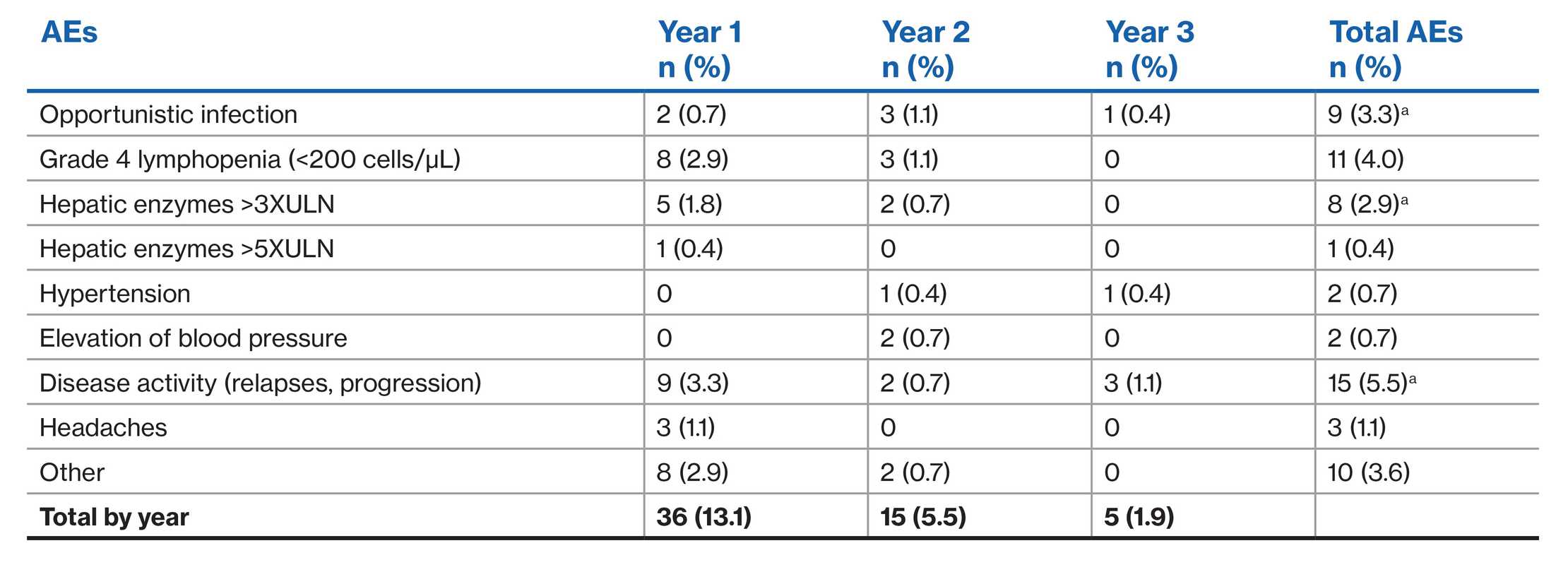
aThree opportunistic infections, one hepatic enzymes>3×ULN and one disease activity could not be assigned to a specific year given the dates are lacking
