COVID-19
Last updated: November 2023. The page will be updated once a year
It looks like you are using an older version of Internet Explorer which is not supported. We advise that you update your browser to the latest version of Microsoft Edge, or consider using other browsers such as Chrome, Firefox or Safari.
Last updated: November 2023. The page will be updated once a year
Novartis routinely monitors cases of Covid-19 in patients on therapy with fingolimod. Based on the totality of the data available from the COVID-19 case reports in clinical trial and post-marketing setting as well as comprehensive data analysis by the MS Data Alliance Global Data sharing initiative1

aThis section provides a summary of cases of fingolimod-treated patients either suspected as having or reported to have COVID-19 as reported in the Novartis safety database, including spontaneous reports submitted voluntarily and cases identified in the scientific literature. There is typically underreporting in this setting; therefore, the true numerator is unknown. The denominator is also unknown as the actual number of patients on therapy with fingolimod is not readily available. Many of the cases contain very limited information, and cases lost to follow up are included. Therefore, due to these limitations, it is not possible to draw any meaningful conclusions concerning the incidence of COVID-19 or course of illness in patients receiving fingolimod.
COVID-19 infection confirmed cases2,3
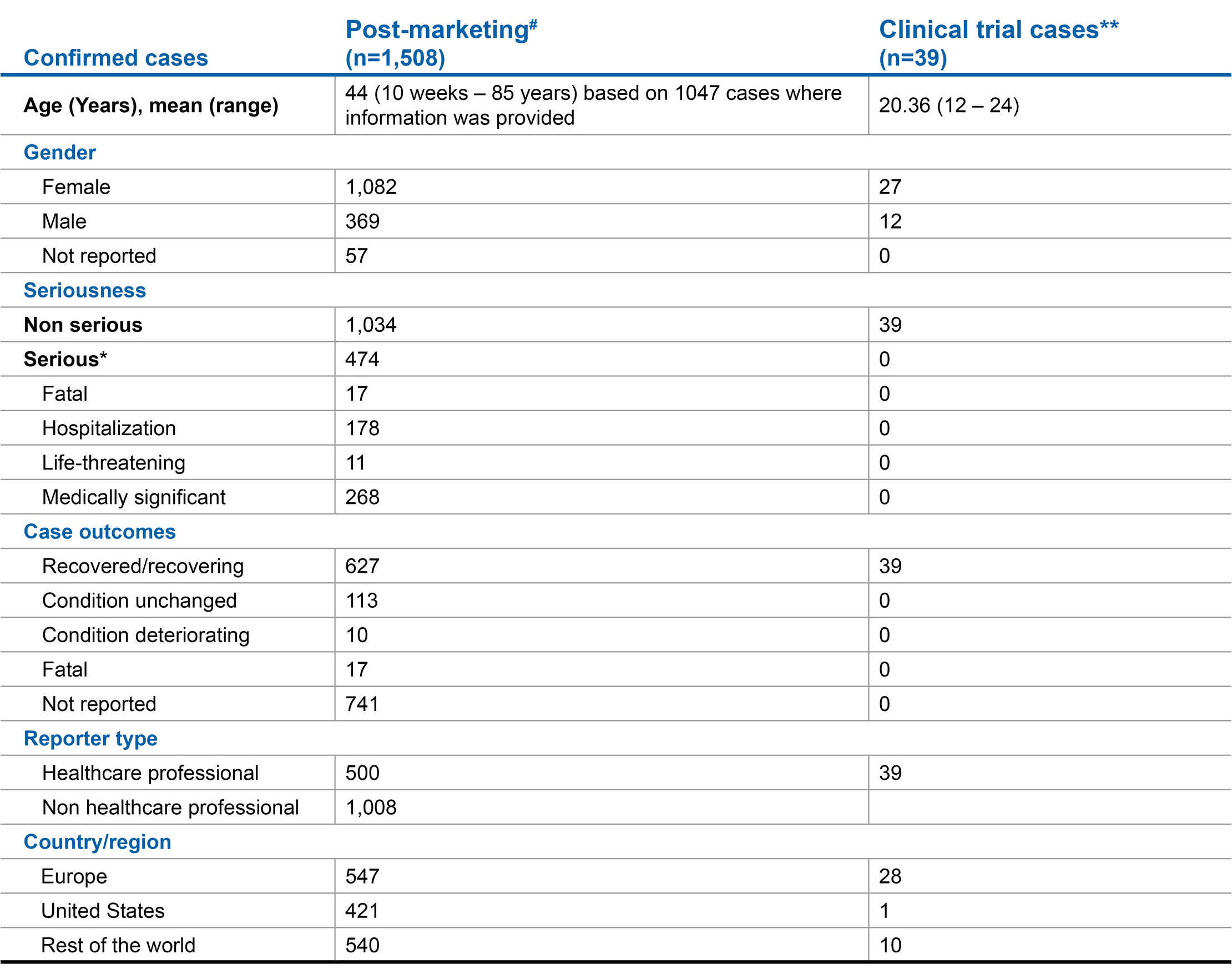
Impact of COVID-19 in MS in a real world setting

This website is for non-promotional purposes and is intended for providing
safety information for healthcare professionals (HCP) only
Please confirm that you are an HCP
For HCPs: Information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Novartis representative for the latest information specific to your country.
For non-HCPs / patients: This safety website is available for HCPs only