FREEDOMS
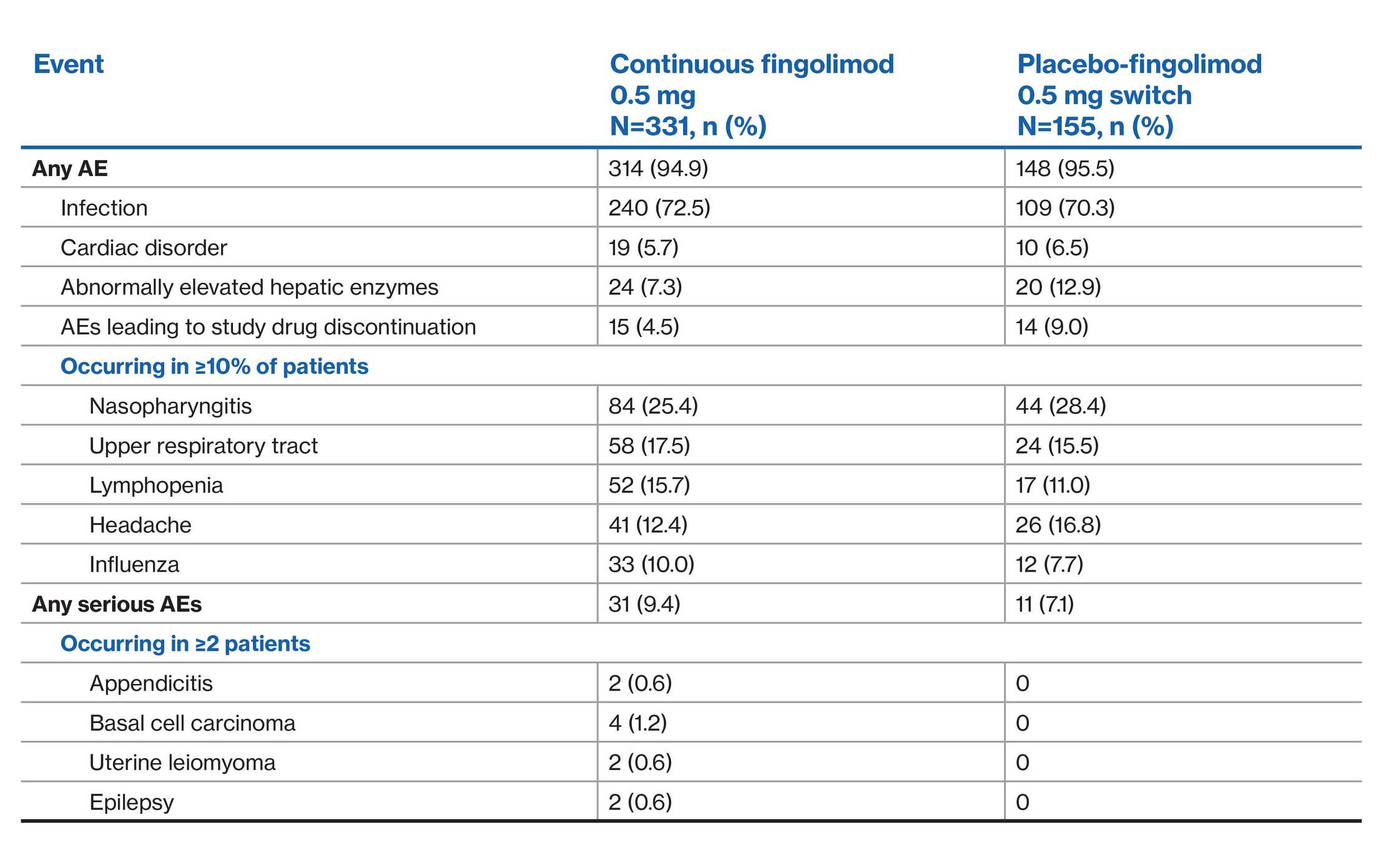
Study design
Double-blind, randomized, placebo-controlled two-year Phase 3 study in adults with RRMS
A 2-year, randomized, double-blind, placebo-controlled phase 3 study of 1,272 adults with RRMS
Patients were randomly assigned to receive a once-daily dose of fingolimod 0.5 mg (n=425) or 1.25 mg (n=429) or matching placebo once daily (n=418) for 2 years
Patient baseline characteristics:
1. Between 18 and 55 years of age
2. A diagnosis of RRMS with at least 1 documented relapse during the previous year or at least 2 documented relapses during the previous 2 years
3. A score of 0.0 to 5.5 on the EDSS. Median score at baseline was 2.0
Primary end point: ARR
Key secondary end point: time to 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5) sustained for 3 months**
Additional secondary end points included number of Gd+ T1 lesions and number of new or newly enlarged lesions on T2-weighted MRI scans
**The analysis of a key secondary end point includes the same intent-to-treat population as the primary end point(s), as well as logistic regression adjusting for certain baseline characteristics of the sample
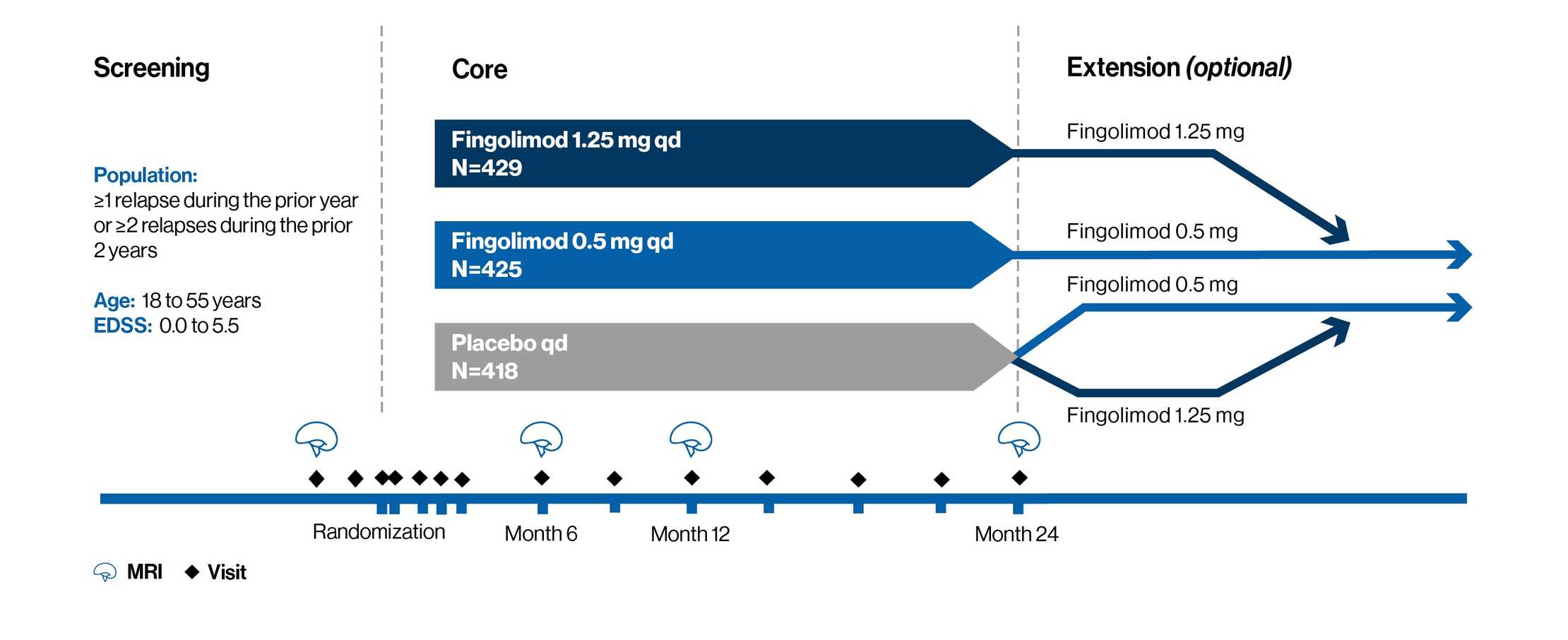
AE profile of fingolimod versus placebo over 2 years: FREEDOMS and FREEDOMS II studies1
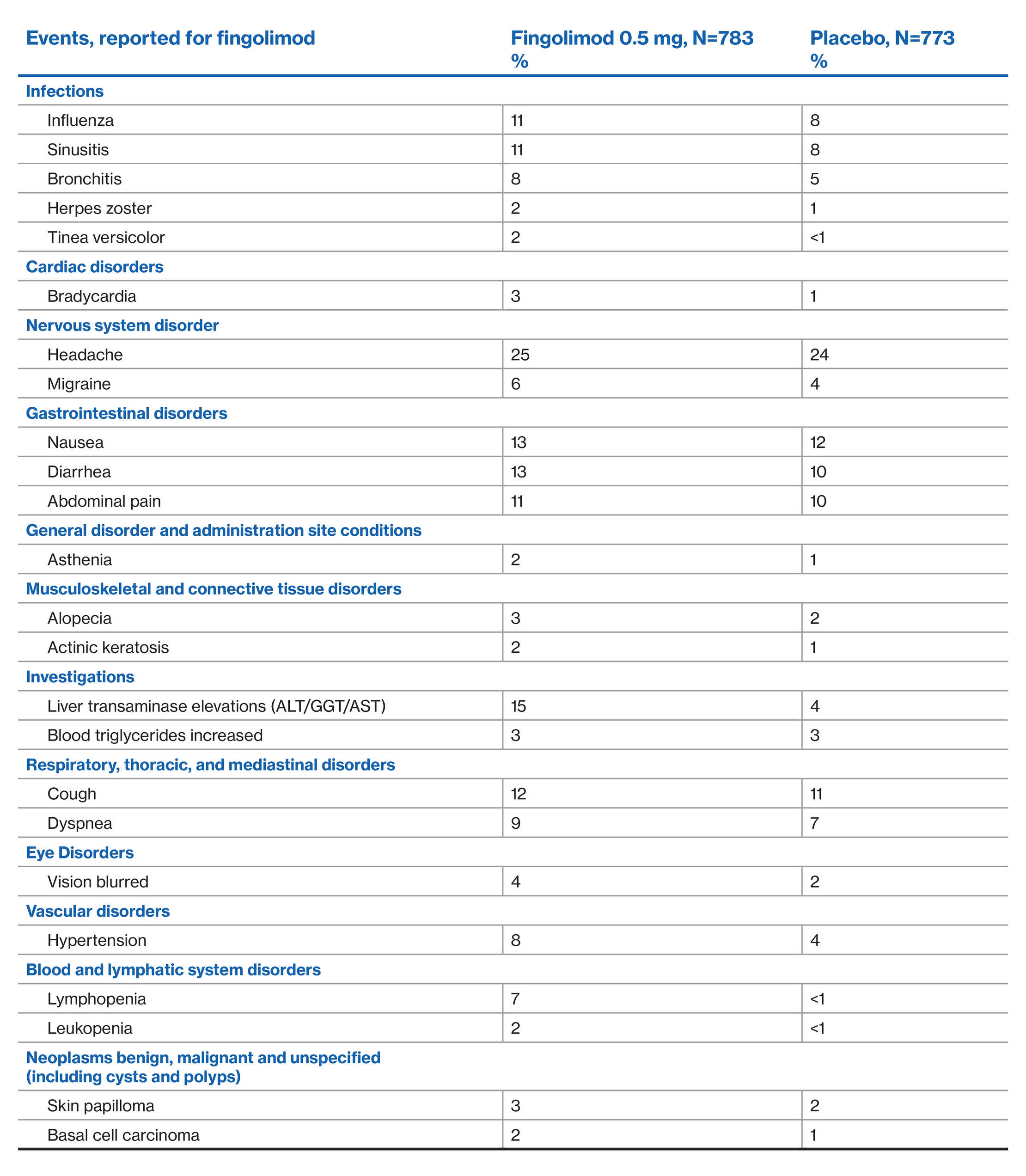
AE profile of fingolimod over 4 years: FREEDOMS Extension study2