ASSESS
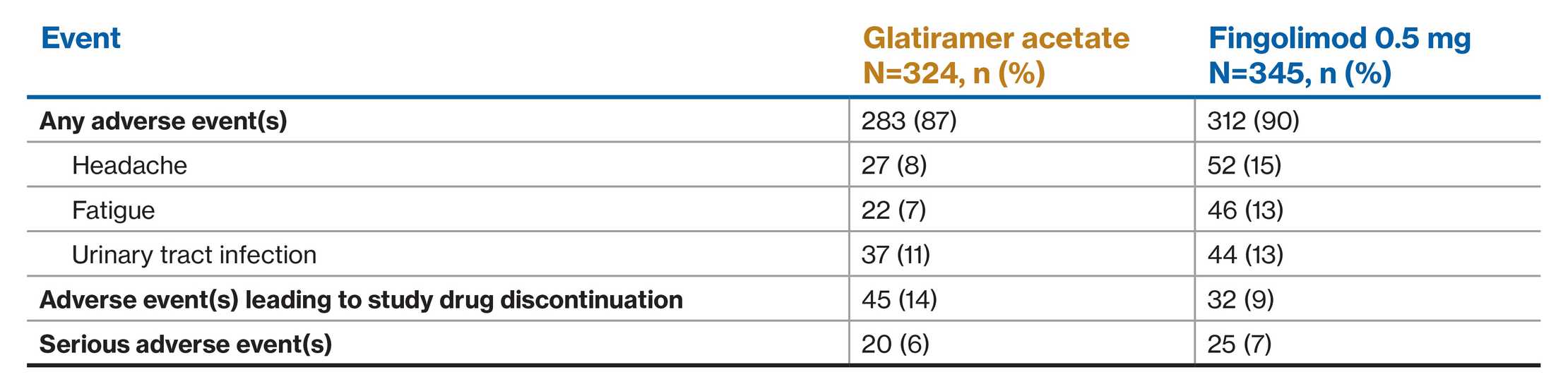
Study design
Dose- and rater-blinded, randomized, active-controlled, parallel-group, one-year Phase 3b study in adults with RRMS
EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple scierosis; s.c.subcutaneous Bruce Cree, et al Efficacy and safety of Fingolimod 0.5 mg and 0.25 mg Versus Glatiramer Acetate 20mg in Patients with Relapsing-Remitting Multiple Scierosis - ASSESS study Group.
Neurology Apr 2019, 92 (15 Supplement)S56.009
A 1-year, randomized, dose-blind, rater-blind, active-controlled (glatiramer acetate injection) SQ phase 3b study in adults with RRMS. Patients randomly assigned to receive once-daily fingolimod 0.25 mg and 0.5 mg vs once-daily subcutaneous injections of glatiramer acetate 20 mg. At baseline, patients with RRMS between 18 and 65 years of age (median 39) had ≥1 documented relapse during the previous year or ≥2 documented relapses during the previous 2 years, and had a score of 0.0 to 6.0 on the EDSS (median score at baseline was 2.5). The primary end point was ARR
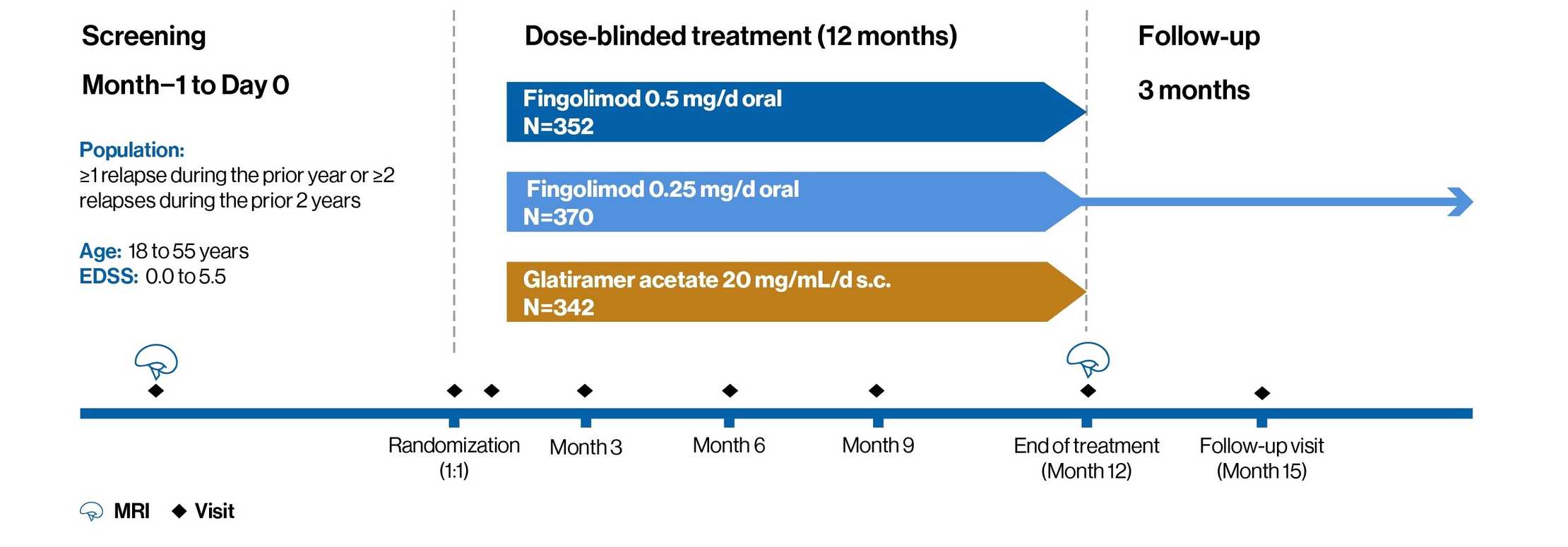
AE profile of fingolimod versus glatiramer acetate: ASSESS study1