PANGAEA
PANGAEA is a prospective, multi-center, non-interventional study of fingolimod, conducted in Germany, to investigate long-term safety and tolerability of fingolimod in daily clinical practice in patients with RRMS (N=4019)
Incidence of AEs and SAEs1
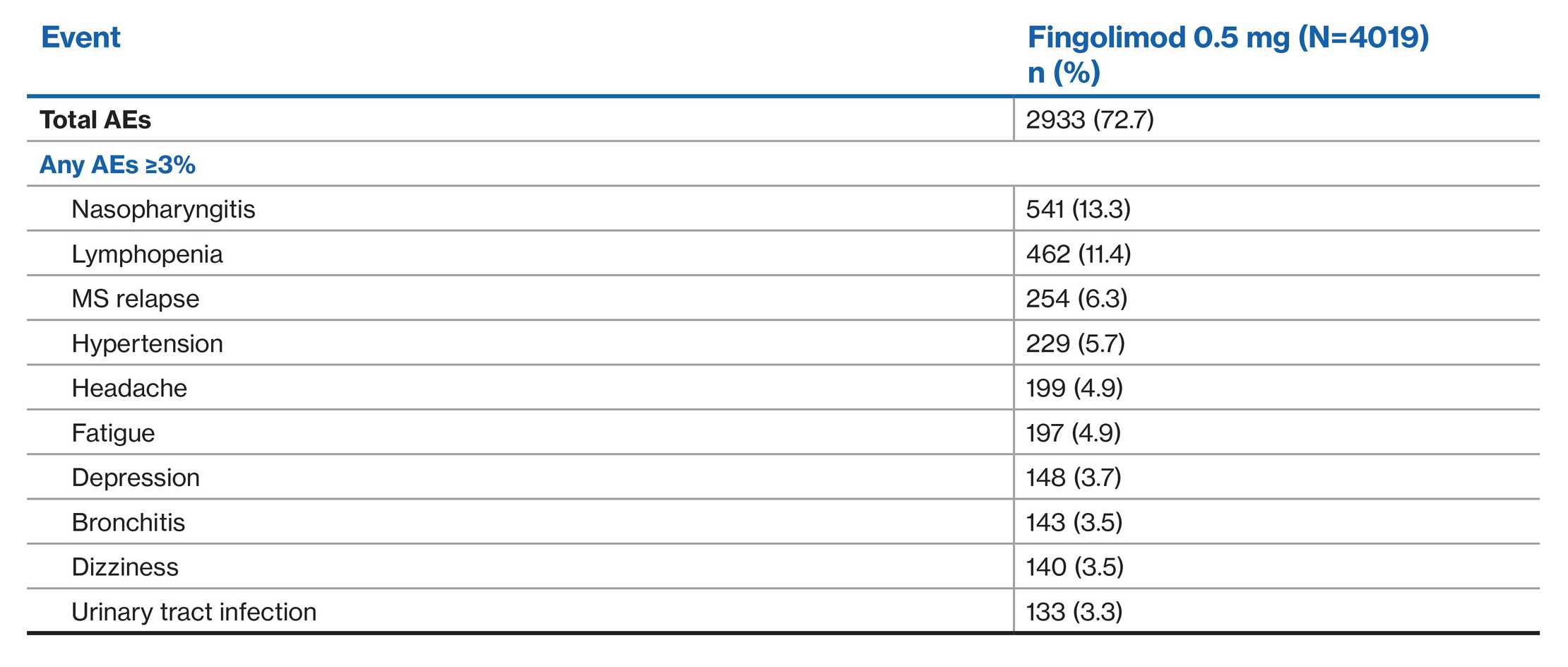
Study discontinuation due to AEs1
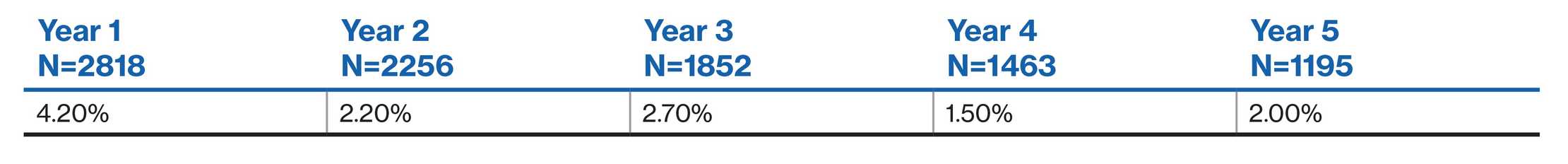

Incidence of AEs and SAEs: Switch from other DMTs2
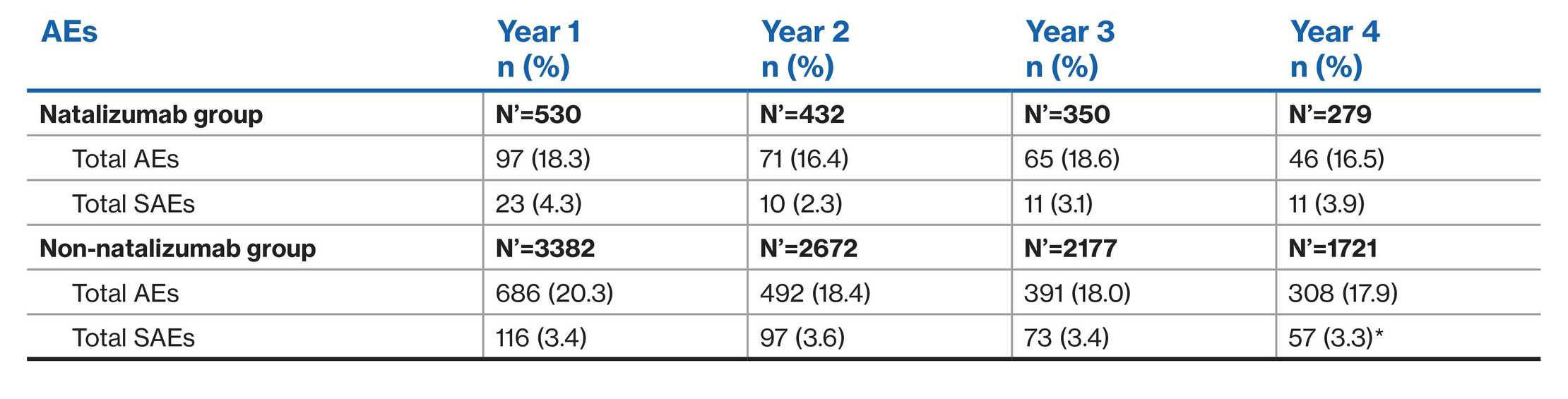
Natalizumab group – Most recent DMT at baseline was natalizumab
Non-natalizumab group – Most recent DMT at baseline was not natalizumab
*One case of PML
