Progressive multifocal leukoencephalopathy
- Cases of PML have occurred in patients with MS who received fingolimod in the post-marketing setting1
- The overall incidence of PML under fingolimod therapy not attributed to previous natalizumab treatment is very rare and the risk remains low
- The risk factors for developing PML in fingolimod-treated patients is not yet established
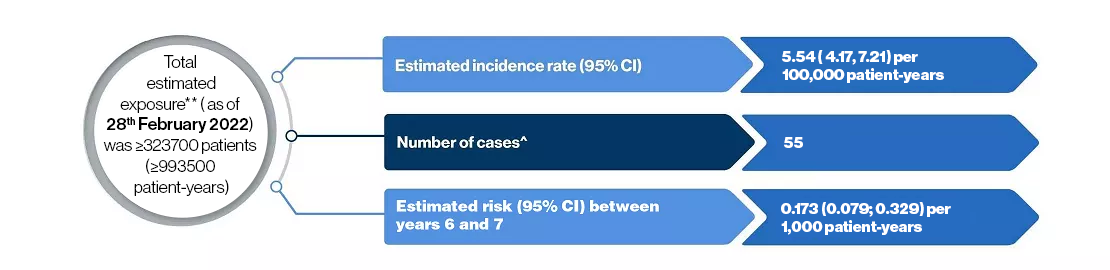
**Total exposure includes both commercial and clinical trial exposure
^Cases under fingolimod therapy not attributed to previous natalizumab treatment
Case details of 55 patients who developed PML under fingolimod
- Mean age at the time of PML diagnosis was 54 years
- There were 43 female and 12 male patients
- Based on available ALC information, none exhibited sustained grade 4 lymphopenia (<200 cells/ul)

- The incidence of PML appears to increase with treatment duration. However, the exact pattern of the relationship to duration of treatment is unclear due to wide 95% confidence intervals
- Between 6 and 7 years, the estimated risk is 0.173 (0.079; 0.329) per 1000 patients
Incidence of PML per 1,000 patients by treatment duration#
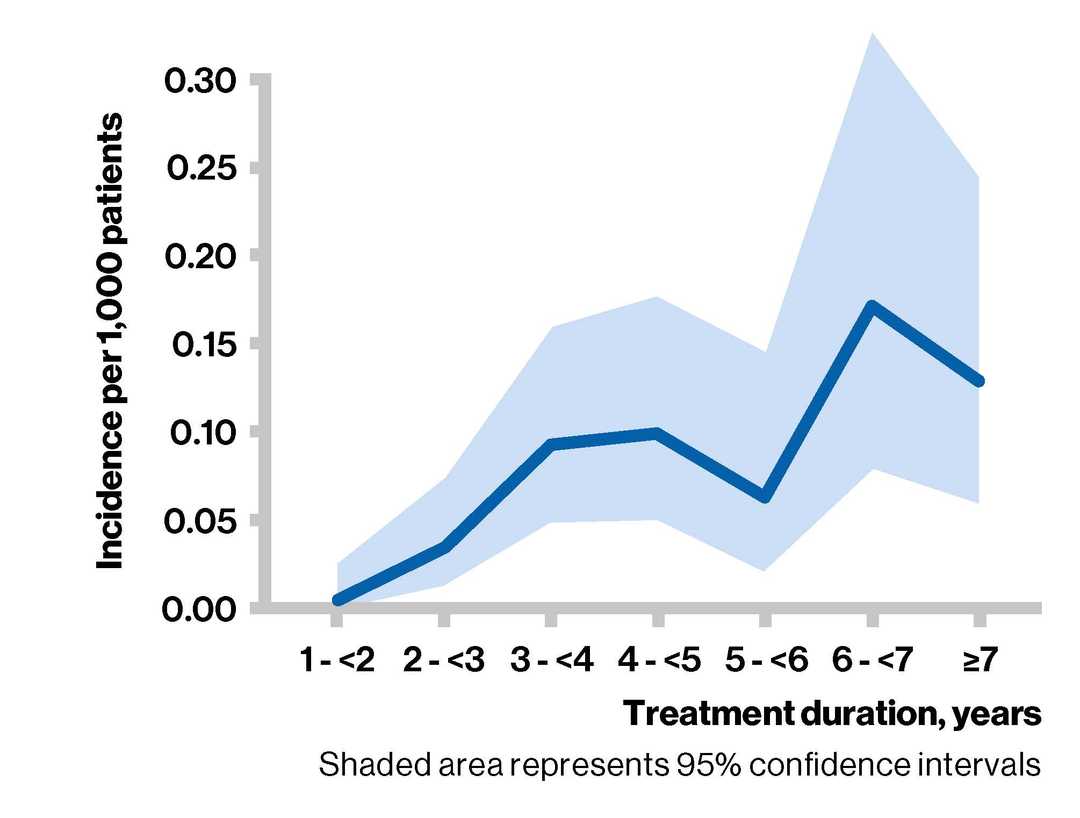
#For each period, the number of PML cases attributed to fingolimod was divided by the corresponding exposure to estimate the incidence (hazard) within each period. Data beyond Year 7 were scarce and hence years 8,9 and 10 were pooled.

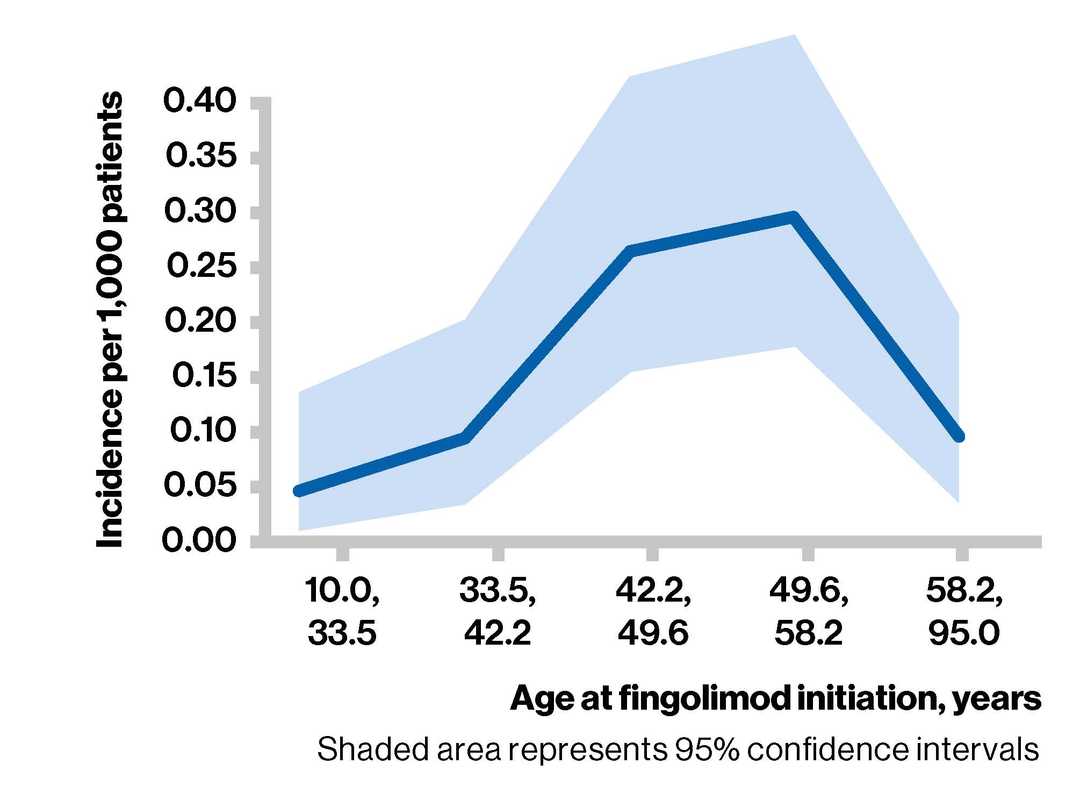
- The exact shape of the relationship with age is unclear due to wide 95% confidence intervals, uncertainties around underlying assumptions, and other unknown confounding factors
Incidence of PML per 1,000 patients by age at fingolimod initiation
About PML
About PML
- Progressive multifocal leukoencephalopathy is caused by reactivation of the JC virus, a ubiquitous human papovavirus that is typically acquired during childhood and remains latent in the kidneys and possibly other sites (eg, mononuclear cells, CNS)
- Increasingly, PML is occurring as a complication of immunomodulatory therapy
- Common symptoms/signs include clumsiness, hemiparesis, aphasia, dysarthria, hemianopia and cognitive impairment
- PML is suspected in patients with unexplained progressive brain dysfunction, particularly in those with depressed cell-mediated immunity
- CSF is analyzed for JC viral DNA using PCR; a positive result with compatible neuroimaging findings is nearly pathognomonic
- Supportive management and management of underlying disorders are helpful in managing PML
Suspected or confirmed PML: Get Novartis support
Novartis support
- Novartis can help physicians with the assessment of a suspected PML case, after the adverse event has been reported to the company via standard pharmacovigilance processes
- Subsequently, there is access to an external expert MRI service and Novartis has constituted an external PML adjudication panel for case evaluation
- If a second opinion on an MRI with atypical findings/suspicion of PML is requested, Novartis can support obtaining this through a MRI expert center (MIAC), University Hospital Basel, Switzerland. Novartis will not have access to the MRI and will only receive a report
- In addition, Novartis can support logistics for shipment of CSF samples for testing for presence of JCV DNA (PCR) at Unilabs A/S, Denmark. In this case, both the physician and Novartis receives the report

Last updated: February 2022. The page will be updated twice a year.