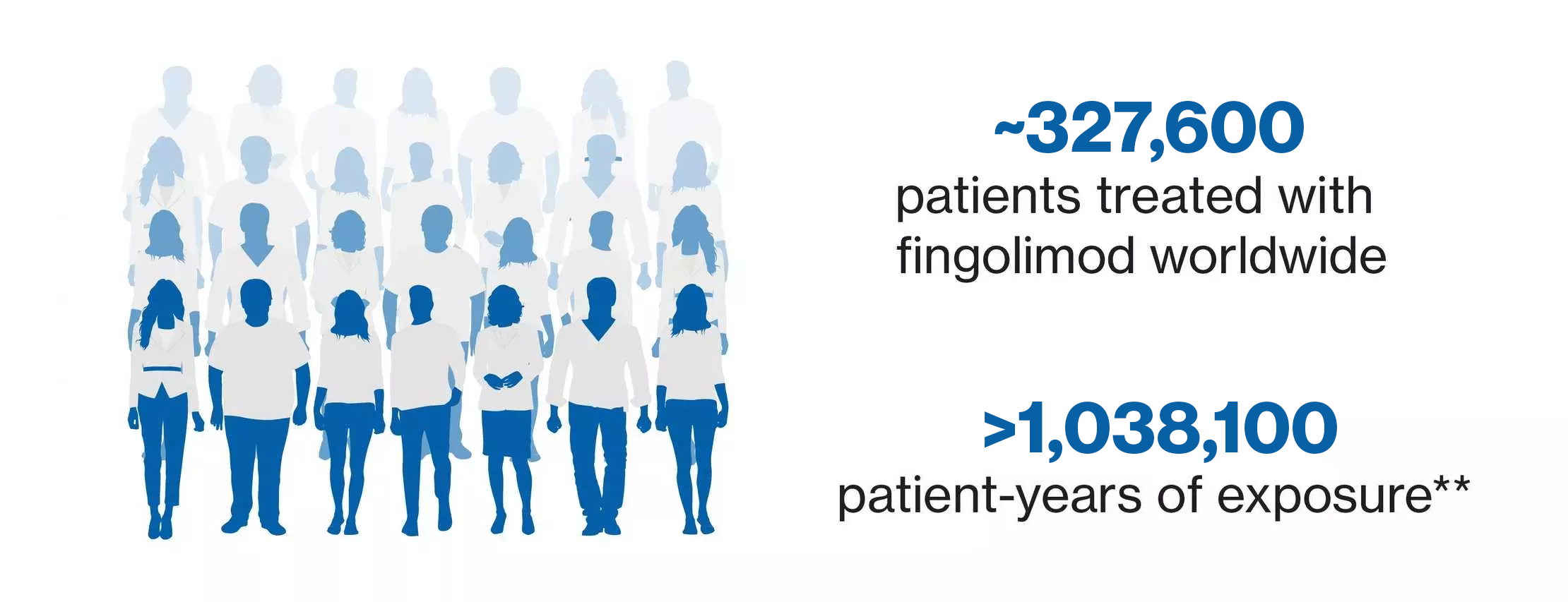
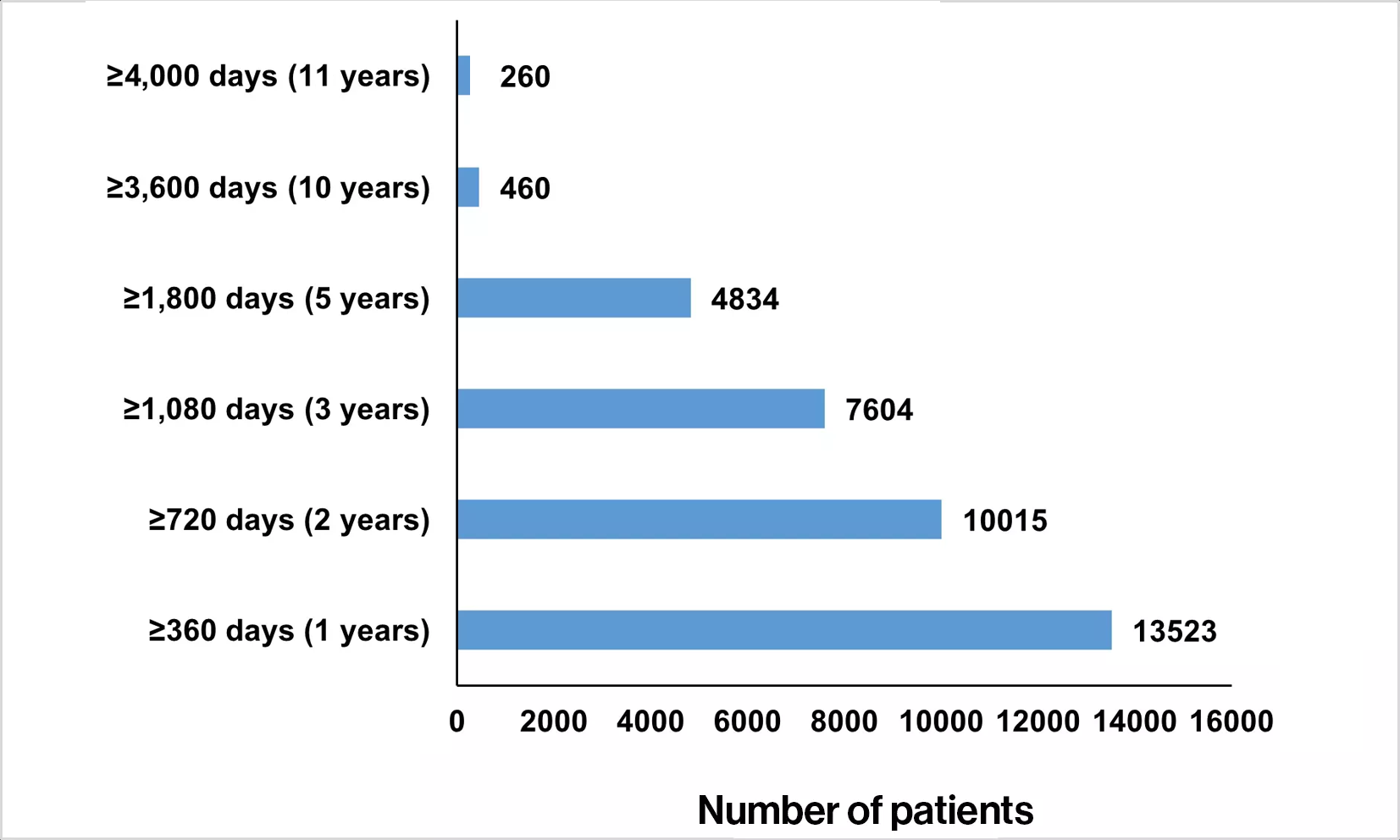
Our commitment to ongoing communication on safety
A verified up-to-date global source of safety information from clinical trials and
postmarketing experience with fingolimod for healthcare professionals
Full content last update: August 2021

Our commitment to ongoing communication on safety
A verified up-to-date global source of safety information from clinical trials and
postmarketing experience with fingolimod for healthcare professionals
Full content last update: August 2021

Our commitment to ongoing communication on safety
A verified up-to-date global source of safety information from clinical trials and
postmarketing experience with fingolimod for healthcare professionals
Full content last update: August 2021