References
1. Simpson-Yap S, et al. medRxiv 2021.02.08.21251316; doi: https://doi.org/10.1101/2021.02.08.21251316.
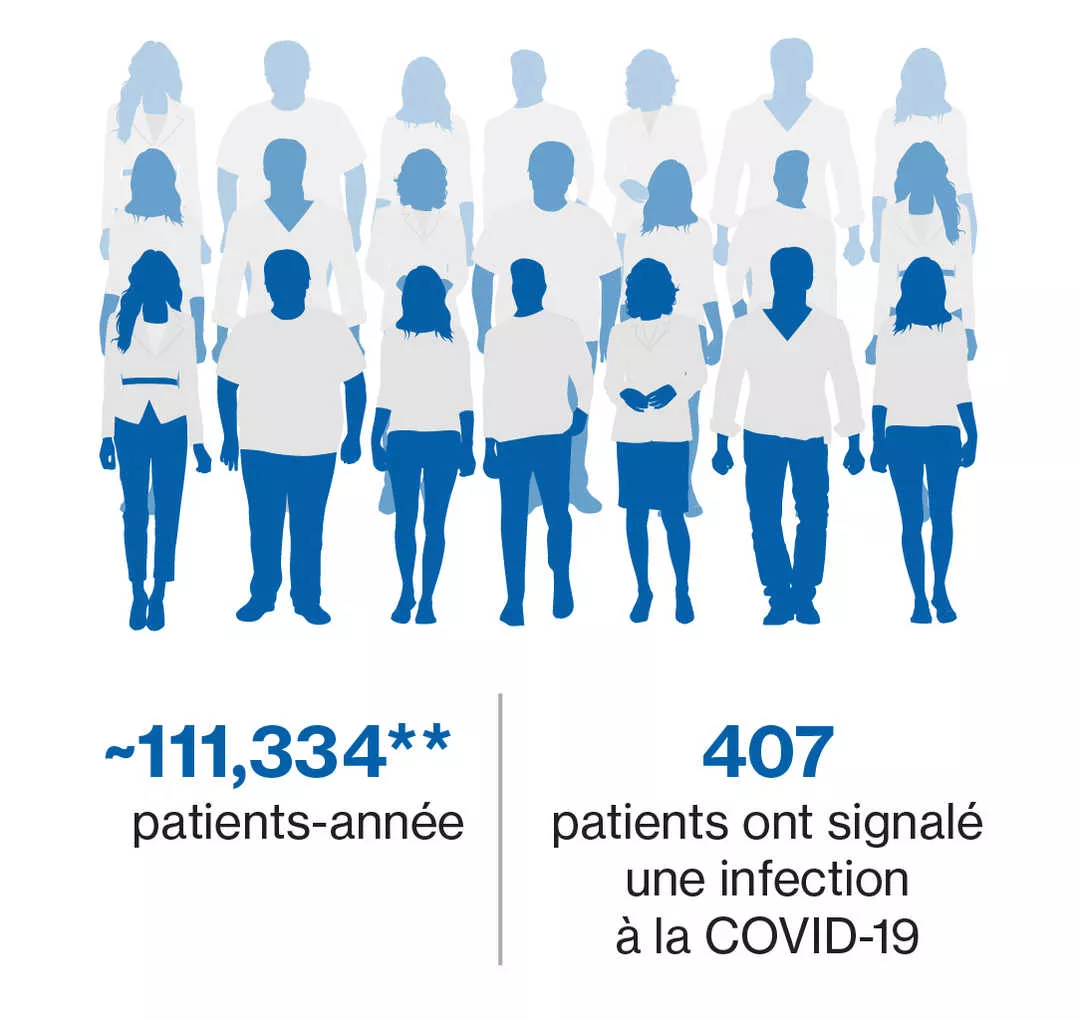
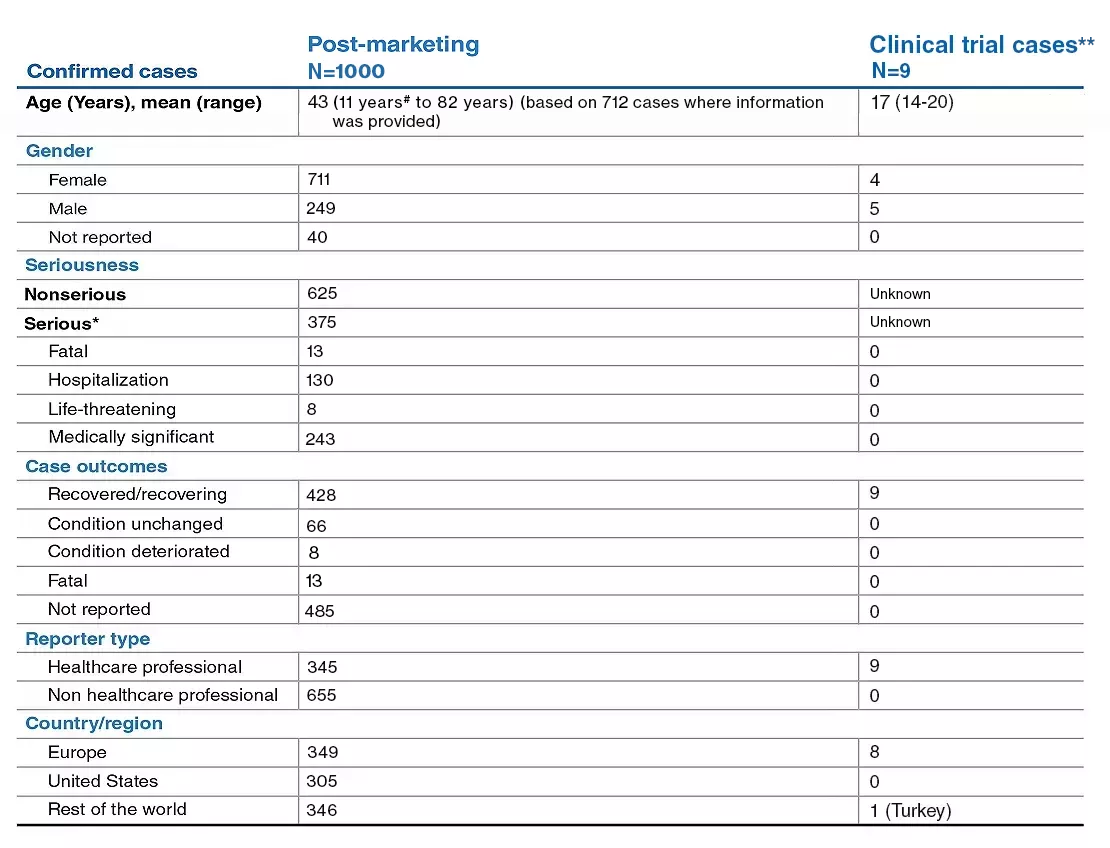
2. Data on File, Novartis safety database, cutoff date 28th February 2022. Novartis Pharma AG.
3. Data on File, Novartis safety database, cutoff date 04-August-2021. Novartis Pharma AG.
4. Oh J, et al. Curr Opin Neurol. 2018;31(6):752-759.
5. Rostami Mansoor S, et al. J Med Virol. 2021;93:1314-1319.
6. Reder A.T et al. CNS Drugs (2021) https://doi.org/10.1007/s40263-021-00804-1
7. Sormani MP, Salvetti M, Labauge P et al. DMTs and COVID-19 severity in MS: a pooled analysis from Italy and France. Annals of Clinical and Translational Neurology 2021.
doi:10.1002/acn3.51408
8. MS International Federation. Global COVID-19 advice for people with MS. Last updated on 4 June 2021. Accessed 19-Nov-2021.
http://www.msif.org/wp-content/uploads/2021/06/June-2021-MSIF_Global-advice-on-COVID-19-for-people-with-MS_FINAL.pdf
9. World Health Organization. Coronavirus disease (COVID-19) outbreak. Accessed 4-Dec-2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
10. European Centre for Disease Prevention and Control. COVID-19. Accessed 4-Dec-2020. https://www.ecdc.europa.eu/en/novel-coronavirus-china
11. US Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Accessed 4-Dec-2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinicalguidancemanagement-
patients.html#clinical-management-treatment%3C
12. Ziemssen T, et al. Poster presented at ECTRIMS 2021; P810
13. Rauser B et al. AAN 2022, April 24-26, 2022. https://index.mirasmart.com/aan2022/PDFfiles/AAN2022-001304.html
14. Sormani MP et al. Oral presentation at ECTRIMS 2021
15. Weinstock-Guttman B et al. Poster presented at ECTRIMS 2021; P630
16. Ahmad Z Mahadeen et al. Poster presented at ECTRIMS 2021; P978
17. Breakthrough Infections: Coronavirus After Vaccination | Johns Hopkins Medicine. Accessed 25 November 2021.
18. Stærke NSea. Levels of SARS-CoV-2 antibodies among fully-vaccinated individuals with delta or omicron variant breakthrough infections: a prospective cohort study. Accessed on 23rd May 2022. Lancet (preprint). 2022
19. Sormani MP, Schiavetti I, Inglese M, et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine 2022. doi:10.1016/j.ebiom.2022.104042
20. Fingolimod (Gilenya®) EU Summary of Product Characteristics. Last updated 3-August-2021. Accessed on 6-December-
2021 https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya
21. Ufer M, et al. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):e398.
22. Novartis Data on File. EXPAND core CSR Novartis Pharmaceuticals Corp.
23. Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. Updated February 10, 2021.
Accessed February 10, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
24. National Multiple Sclerosis Society.MS, the coronavirus and vaccines – updated global advice. Accessed May 20, 2022. The coronavirus and MS – updated global advice (msif.org)
25. US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. Accessed November 20, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised
26.United Nations. WHO advisory group recommends extra COVID-19 vaccine dose for immunocompromised. Accessed November 20, 2021. https://news.un.org/en/story/2021/10/1102732
27. Reuters. Factbox - Countries weigh need for booster COVID-19 shots. Accessed October 27, 2021. https://www.reuters.com/article/us-health-coronavirus-booster-idUKKBN2GA190
28. Mayzent Prescribing information. Novartis Pharmaceuticals Corp; 2020
29. National Multiple Sclerosis Society. Timing MS medications with COVID-19 mRNA vaccines. Accessed February 18, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-
information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance/Timing-MS_Medications-with-COVID-19-mRNA_Vaccines